Introduction: Myeloproliferative neoplasms (MPN) are clonal disorders caused by mutations that activate JAK/STAT signaling through the thrombopoietin receptor, MPL, leading to overproduction of myeloid lineages. MPL is the only hematopoietic growth factor receptor expressed on the surface of hematopoietic stem cells (HSC) where it maintains their quiescence by tethering HSCs to osteoblasts in the endosteal niche. MPL is also expressed on megakaryocytes and platelets. Individuals born with loss of function mutations in the distal MPL cytokine receptor homology domain (CRHD) develop thrombocytopenia and progressive bone marrow failure. How MPL functions in MPN remains unclear. Here, we describe a novel MPL splice variant ( MPL-SV) that is present in CD34 + cells and platelets from healthy controls, but overexpressed in the MPN. We therefore sought to: 1) define MPL-SV function in hematopoiesis and MPN, and, 2) elucidate mechanisms underlying MPL-SV.
Methods: After identifying a novel MPL-SV transcript from JAK2 V617F MPNplatelets, we compared its expression to MPL transcripts lacking the deletion in MPN patients and healthy controls. To assess its function, we generated transgenic (Tg) mice expressing varied levels of MPL-SV in HSC. Next, we examined responses of the Tg mice to thrombopoietin (THPO), hematopoietic stem progenitor cell (HSPC) frequency by flow cytometry, and HSPC function by colony-forming assays (CFA). To elucidate mechanisms, we performed single cell RNA sequencing (scRNAseq) of bone marrow (BM)-derived Lin -, Sca +, c-kit + (LSK) cells followed by pathway analysis.
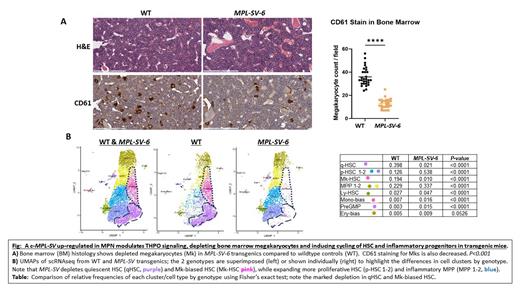
Results: Using primers to amplify the MPL CRHD in platelets from patients with JAK2 V617F MPN, we discovered an MPL- SV lacking 7 amino acids in the MPL amino-terminal domain, a common hotspot for activating MPN mutations. MPL-SV is present in CD34 + cells and platelets of healthy controls, but overexpressed in MPN relative to MPL transcripts lacking the deletion. To examine its function, we generated transgenic (Tg) mice expressing MPL-SV in HSC. Tg miceexpressing a single copy of MPL-SV have unperturbed hematopoiesis, whereas Tg mice expressing 6 copies of the transgene ( MPL-SV-6) develop marked thrombocytopenia, mild splenomegaly, and a modest increase in red cells by 9-12 weeks of age. Baseline serum THPO levels are elevated in Tg MPL-SV-6 mice and they fail to increase platelet counts following exogenous THPO administration, suggesting defective function of MPL-SV in binding and/or internalizing THPO. To further assess MPL-SV function, we compared CFA from HSPC of Tg MPL-SV-6 and wildtype mice, which show a decrease in total colony forming units (CFU) from BM or spleen-derived MPL-SV-6 HSPC. Accordingly, there was a decrease in LSK cells, multi-potent progenitors (MPPs), and megakaryocytes (Mk) in BM by flow cytometry. By contrast, spleens show increases in LK cells and short-term HSC. Further analysis by scRNAseq of BM-derived LSK cells reveal a marked decrease in both quiescent HSC and HSC with a Mk-bias associated with a relative expansion in more proliferative HSC, including those with a bias towards erythroid and mature myeloid differentiation. Pathway analysis reveal that MPL-SV activates transcriptional networks involved in TNF-α signaling via NF-κB and oxidative phosphorylation. Analysis of cell-cell interactions by CellChat show decreased signaling through intercellular adhesion molecule 1 (ICAM-1) in MPL-SV-6 HSC compared to controls. Importantly, ICAM1 maintains quiescent HSC within their BM niche. Similarly, cell cycle analysis from scRNAseq indicate that quiescent HSC are stimulated to cycle and generate HSC primed to differentiate into erythroid and myeloid progeny.
Conclusions: We discovered a novel MPL-SV that is up-regulated in MPN. In Tg mice, MPL-SV impairs responses to THPO and disrupts hematopoiesis by depleting quiescent HSC and BM megakaryocytes. Single cell transcriptomics suggest that MPL-SV drives expansion of cycling HSC and HSC primed to differentiate into progenitors involved in inflammation, including myeloid cells. Mechanistically, HSPC expressing MPL-SV-6 activate transcriptional networks involved in TNFα signaling via NF-κB pathways. Our results highlight the central role for MPL in hematopoiesis and suggest that aberrant MPL splicing contributes to MPN pathogenesis.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal